TARGIT-A clinical trial shows significant improvements for breast tumor treatment using intraoperative radiation therapy
The latest results from the international TARGIT-A clinical trial have just been published in the International Journal of Radiation Oncology, Biology and Physics*. This is a study focused on long-term local control and survival rates for breast cancer patients treated with TARGIT (intraoperative radiation therapy using the INTRABEAM® system), compared to patients treated with external radiation therapy.
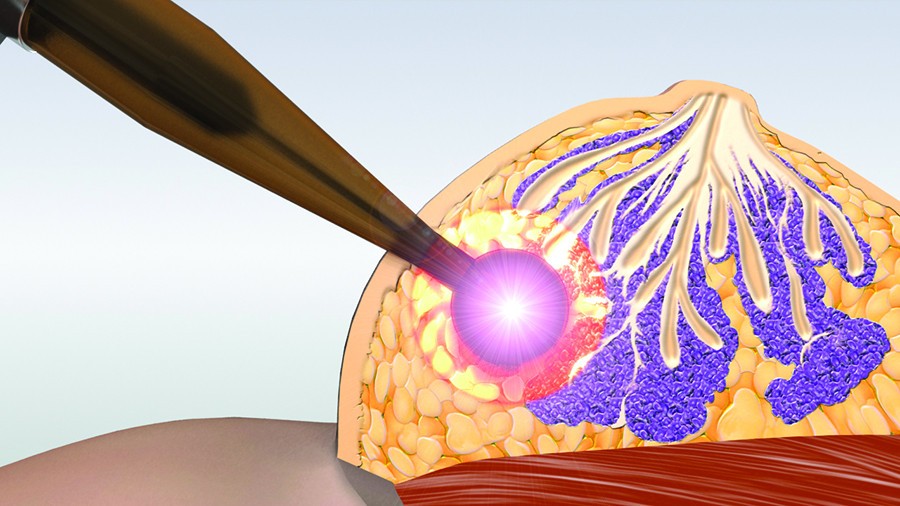
It has been demonstrated that the dispersed radiation that accompanies full breast irradiation during external radiation therapy can induce development of other types of tumors (lung, stomach, etc.), in addition to increasing the risk of heart attacks, especially in smokers. Through treatment with a single, more localized dose, such as by intraoperative radiation therapy using the INTRABEAM® system produced by Carl Zeiss Meditec, which incorporates the Radiance™ planning software from GMV, immediately following a lumpectomy (surgery that removes the cancer and other abnormal breast tissue, along with a small amount of normal tissue surrounding it, but not the breast itself) produces better tumor control efficacy and a lower mortality rate.
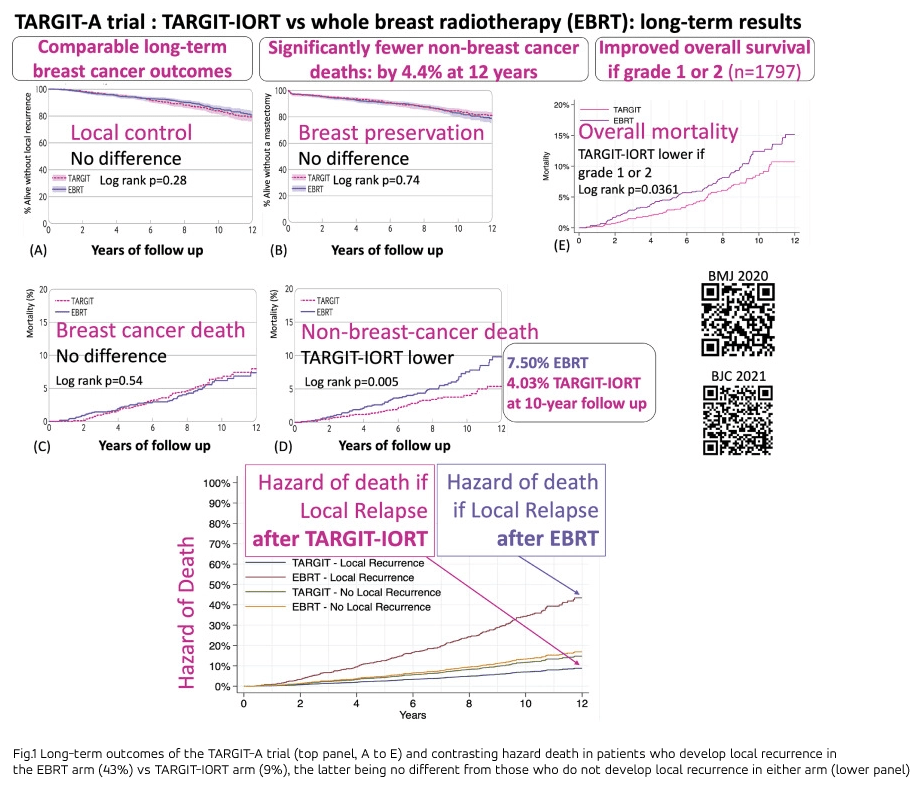
As explained by Carlos Illana, head of Secure e‑Solutions at GMV, «this clinical trial was conducted with 2,298 patients and up to 19 years of follow‑up, with an average of 8.6 years, so the results are considered to be very solid». It is worth emphasizing that when assessing the efficacy of any cancer therapy, it is of special interest to be aware of the progression-free survival rate as well as the overall survival rate. In relation to this, and as demonstrated by the graphs produced during the clinical trial, «with external radiation therapy, the probability of death in the case of local recurrence is a little over four times as high, and the probability of death from causes not due to the cancer is approximately double, compared to treatments performed using intraoperative radiation therapy».
In addition, the trial has concluded that for patients with early-stage breast cancer, targeted, single-dose intraoperative radiation therapy (IORT) applied during the tumorectomy surgery can avoid the toxicity and other undesirable effects that may be caused by radiation therapy applied to the entire breast after surgery (such as with external radiation therapy). Use of IORT also reduces pain, generates improved quality of life, and leads to better results cosmetically. Furthermore, the patient does not need to travel as with conventional treatment (such as when it involves six weeks of daily doses, Monday through Friday). This reduces the patient’s emotional stress, with the corresponding impact on quality of life while also reducing the associated carbon footprint in the patient’s country.
In a detailed analysis of subgroups, the 12-year survival rate was 4.4% higher with TARGIT‑IORT for patients with grade 1 and grade 2 cancer, which includes the majority of patients. For the rest, the survival rate results were comparable in the two groups (but still with the advantages of intraoperative radiation therapy described above).
Multiple initial, non-randomized trials have been published regarding the use of TARGIT‑IORT, with similar results seen in more than 3,000 patients treated using that approach in France, Germany, Denmark, Switzerland, and other countries. In 2019, approximately 260 healthcare centers in 38 countries had treated over 45,000 patients using TARGIT‑IORT.